Commissioning, Qualification & Validation Services (CQV)
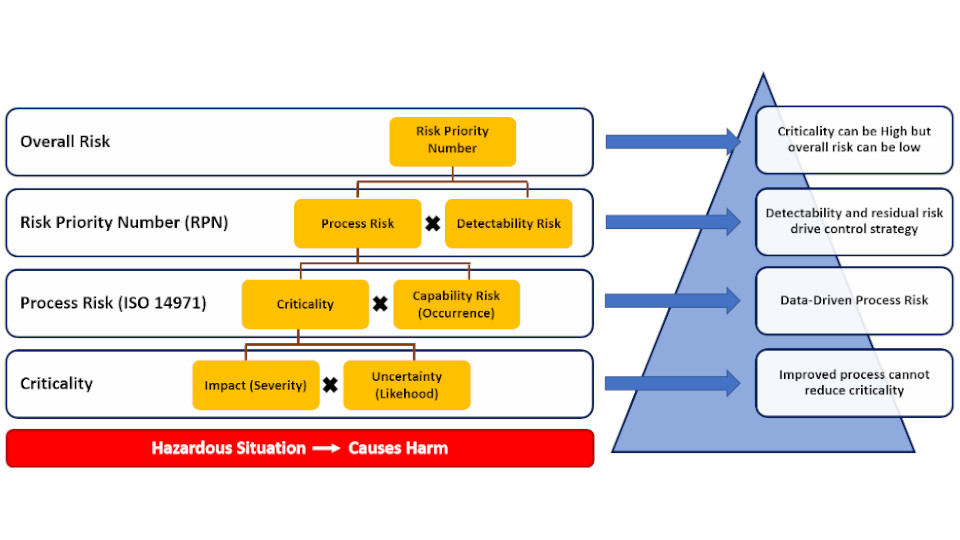
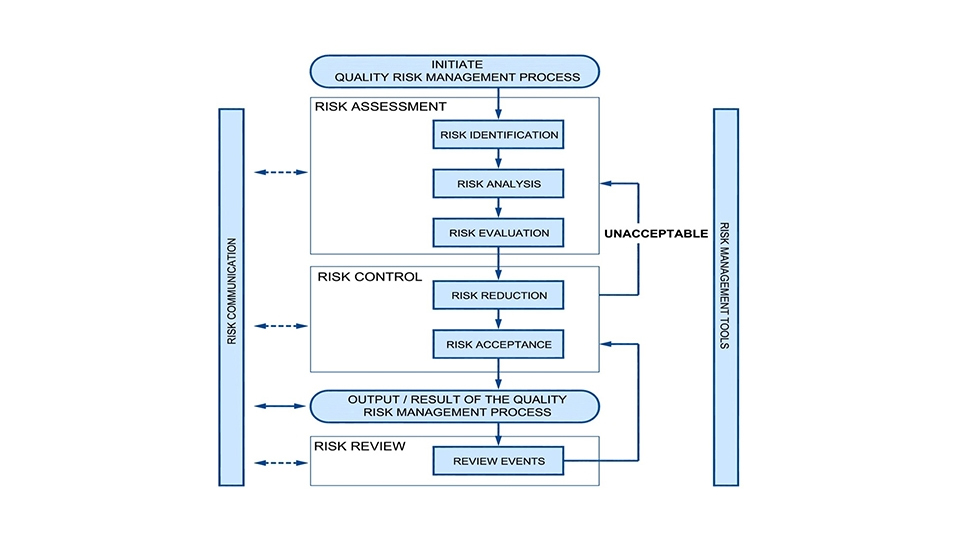
Elomatic follow ICH harmonization guidelines & ISPE Baseline guidelines for the implementation of a risk-based approach for the CQV of Life Science manufacturing facilities, Systems, Utilities and Equipment to validate that they are suitable for the intended purpose. The approach is based on risk analysis, Integrated with focus on product and process.
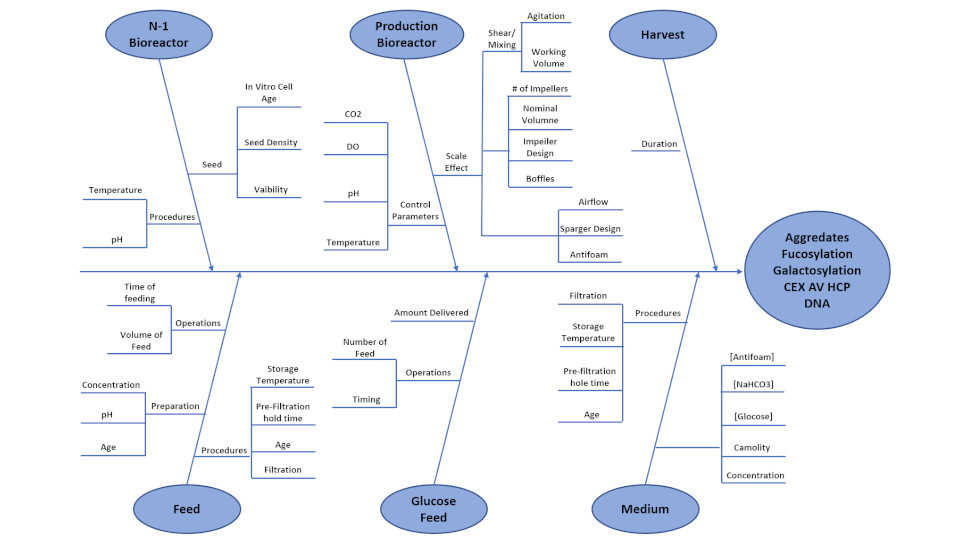
Elomatic follow ASTM E2500 – 13 standard which was drawn up in response to the FDA’s initiative Pharmaceutical cGMP for the 21st Century – A risk-based approach. According to this approach, more emphasis is put on an understanding of the process in the specification, design and verification phases.
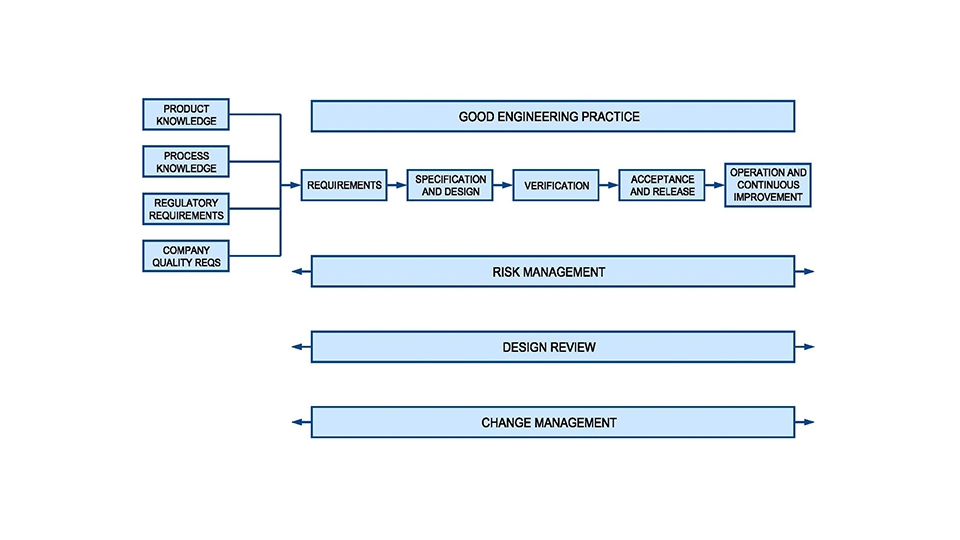
Quality is inbuilt into this process e.g. by relying more on GEP (Good Engineering Practice) and SMEs (Subject Matter Experts) and by applying design reviews as a process throughout the project life cycle instead of it being an isolated event. Elomatic ensures that there are fewer Quality issues and surprises at later stages of investment projects as we take a scientific, proven, proactive, systematic and pragmatic approach to the project.
The User Requirements Specification (URS) is a very important document when planning a new manufacturing, pilot or R&D facility, system or piece of equipment. It is the user’s written thoughts of what is needed. The URS should contain all information necessary (quality, capacity etc.) for a supplier or contractor to be able to suggest a suitable design. The URS forms the basis for all Verification activities and it should be referenced throughout the project life cycle. When handled correctly, verification is an integral part of project management. Elomatic’s focus on projects quality, optimised design & investment and to avoid delays by managing the project as efficiently as possible, e.g. from forward traceability from user requirements (URS) all the way to final verification activities.
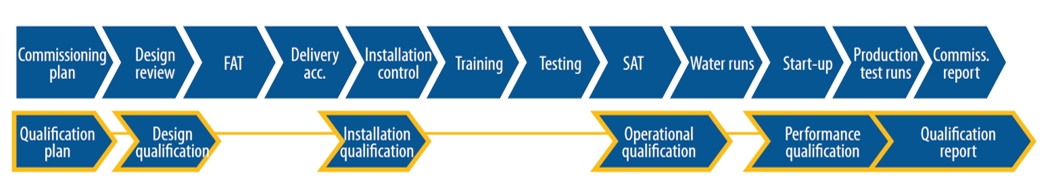
Commissioning is an essential foundation for Efficient Qualification and for further Validation efforts, assuring project success. Elomatic as a leader in these competences, has developed the integrated strategic approach to achieve Qualified Validated Idea to Commercialization..
All the validation deliverables through the Validation Life Cycle (starting with the URS) will focus on product quality and all the testing activities will be used efficiently to give documented evidence that system performance meets products and process user requirements.
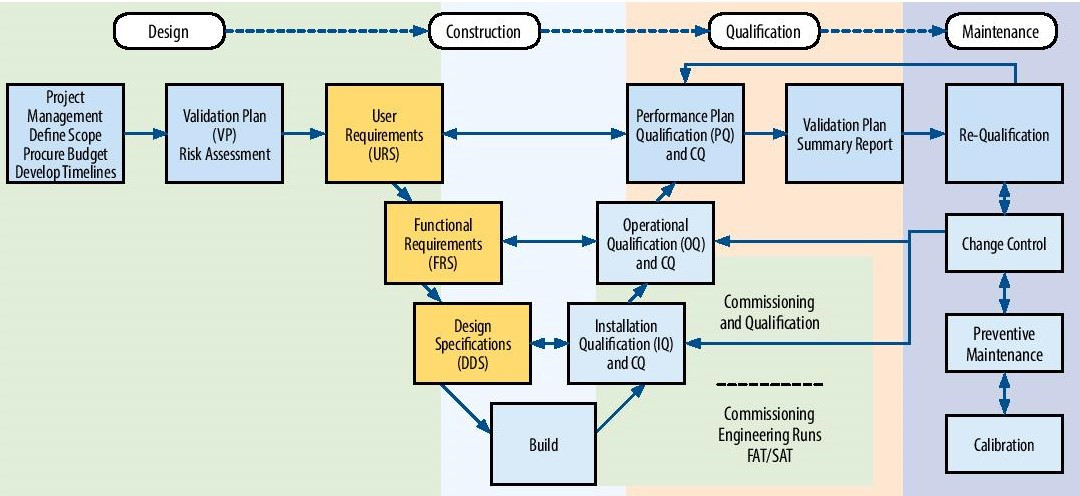
Based on Validation Master Philosophy (VMP) Elomatic assist in preparation, review and finalizations of various qualification protocols (DQ, IQ, OQ, FAT & SAT) of the equipment and support functions. All these Qualification protocols are prepared based on the final approved URS of the facility, system, equipment etc. The qualification execution i.e. execution of IQ and OQ of various equipment at site as per the approved protocols and under the VMP Philosophy shall be carried out.