
Computer System Validation Services (CSV)
Computer System Validation (CSV) is the process of documenting that a computer system meets a set of defined system requirements. Validation of computer systems is to ensure accuracy, reliability, consistent intended performance, and the ability to discern invalid or altered records is a critical requirement of electronic record compliance, as described in the FDA 21 CFR 11.10 (a) and EMA Annex 11, Section 4.
Elomatic follows a Risk-based approach to compliant GxP Computerized Systems “V-Model of GAMP 5”. This is comprehensive system consist of planning, specification, configuration, verification and reporting inclusive of activities like IT infrastructure qualification, periodic review, decommissioning etc. The computerized systems for GxP related processes like production, receipt of raw material storage, dispensing, finished goods despatches, quality control etc. may impact on product quality, data integrity and patient safety.
Therefore the computer system validation must be managed in the framework of a life cycle management according to GMP, GAMP5, FDA 21 CFR Part 11.10 (a) and EMA Annex 11, Section 4.
The risk assessment is carried out at planning stage in order to ensure product quality, data integrity and patient safety.
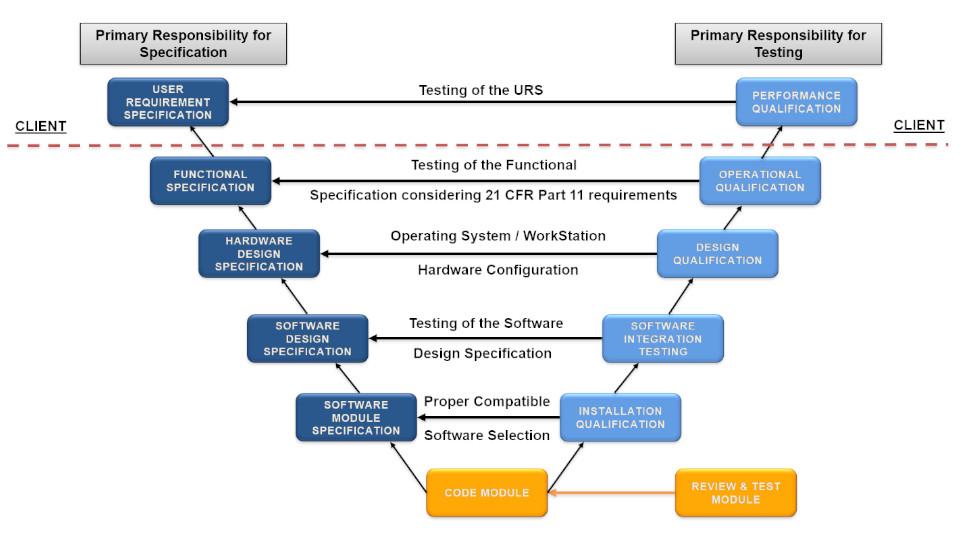
The specifications and procurement of software and hardware is done as per GAMP 5 categories.
Elomatic expert of computer system validation have validated computer programs for all types of FDA-regulated businesses, including pharmaceutical and biologics manufacturers, medical device manufacturers, and GLP laboratories.
Typically, the comprehensive system consist of :
- Computer system validation
- Local control system (SCADA, PLC)
- Plant control system (SCADA, DCS )
- BMS, EMB, FMS
- Laboratory systems (LIMS : stand-alone & networked)
- Management system (ERP, SAP)
- Document management systems
- CAPA systems
- Warehouse management system (WMS)
- e- BMR system
- Serialisation system
- Data integrity process etc.