Containment Engineering in Life Science
Elomatic has proven expertise to Design, Engineer, Qualify & Validate facilities suitable for various high potency drug under several containment classifications. We have credential of building facilities suitable for oncology formulations, hormones, steroids, high potent API as well as bio – containment facilities under BSL2/ BSL3/ BSL4 norms. The containment manufacture requires a specialised approach to design & engineering the manufacturing processes, equipment selection and facility design to achieve desired levels of isolation, containment, minimise operator exposure, ensure worker protection and safety.
A robust containment control strategy is essentials as a growing number of pharmaceutical products contain highly potent active pharmaceutical ingredients (HPAPIs) that are capable of targeting disease selectively and more precisely than any other compounds. HPAPIs are more effective medicines that require lower doses and lead to fewer side effects, but also impose manufacturing challenges due to their toxic, highly potent and sensitising nature that can produce adverse health effects with either acute and chronic exposure.
The concept regarding “Containment” derives from the simple consideration of PPE, process engineering, layout, physical infrastructures and process activities that can isolate the first (product) from the other two elements. Facility design, isolation technologies and general production practices can be more restrictive with respect to handling procedures compared to those employed in typical formulation or API manufacture.
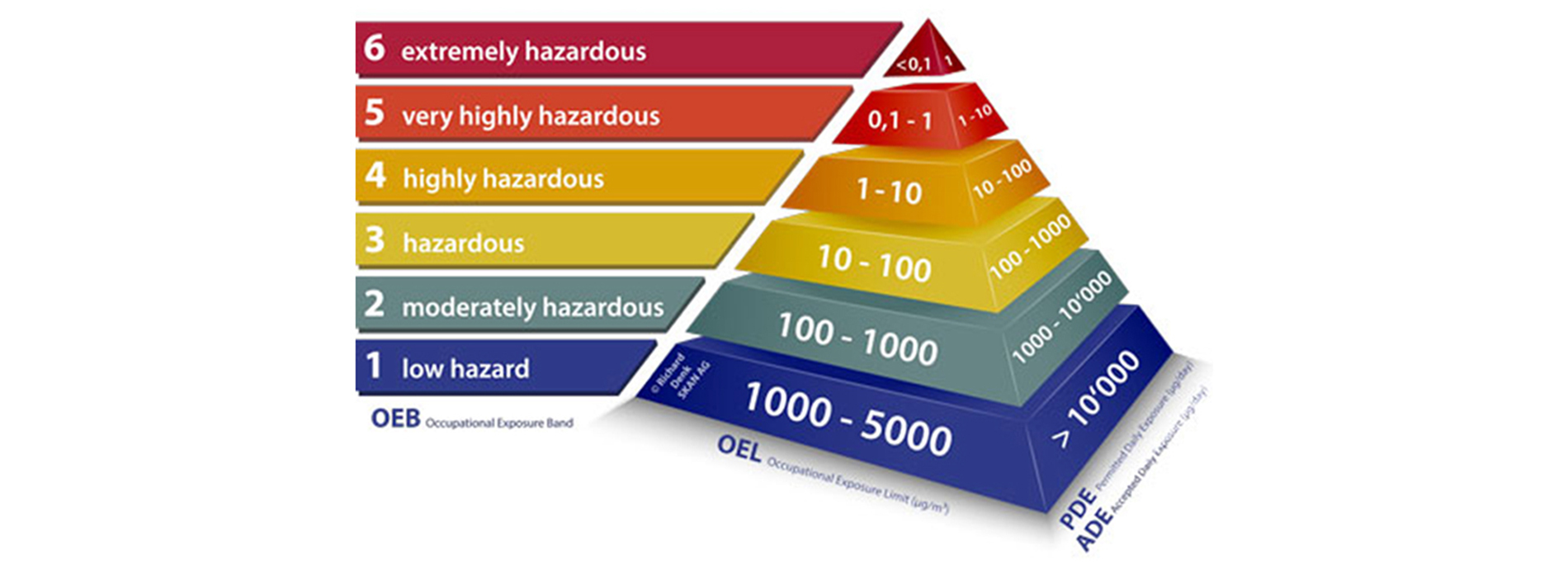
An Occupational Exposure Limit (OEL) is typically set by competent national authorities and enforced by legislation to protect occupational safety and health. It is an important tool in risk assessment and in the management of activities involving handling of dangerous substances. There are many dangerous substances for which there are no formal occupational exposure limits (OEL). In these cases, hazard banding or control banding strategies can be used to ensure safe handling.
The established OEL play vital role in creating the containment philosophy and overall design & engineering of the facility, followed by the procedure protocols etc.
From design & engineering point of view, more emphasis is put on overall design & engineering philosophy inclusive of air lock design, HVAC design, personal protective equipment, gowning philosophy, automation, robotics, drain & ETP, selection of equipment, qualification documents, clean utilities, special civil structure design in case of BSL3/ BSL4 facility etc.
There are multiple philosophies adopted by an pharmaceutical organization for manufacturing a potent drug, viz elimination, substitution, modify process, containment, isolation, ventilation/ extraction, standard operating procedures, plant design etc.


